The need for modern medicine to extend quality life of a worldwide aging population requires new technologies and models for testing new therapies or devices. Besides performing high quality research the CMCiB develops innovative tools and provides expertise to help researchers and industry users transfer their innovations to the patient or the market.
Success stories

A study links the protein HDAC11 as a key factor in muscle deterioration in Duchenne muscular dystrophy
A preclinical study led by the Germans Trias i Pujol Research Institute (IGTP), in collaboration with the Institut de Myologie and the Sant Pau Research Institute, has analysed the role of the protein HDAC11 in Duchenne muscular dystrophy (DMD) and its potential as a therapeutic target.
Read more
HistoSonics develops a groundbreaking non-invasive ultrasound-based Histotripsy Platform to transform liver tumour treatment
The groundbreaking histotripsy therapy platform, developed by the US medical device company HistoSonics, Inc.(USA), uses focused ultrasound to liquefy and destroy targeted liver tumours under real-time image guidance, without the invasiveness or toxicity of traditional oncological treatments.
Read more
The AELIX Therapeutic vaccine as a potential HIV cure strategy, with essential support of CMCiB
A therapeutic HIV vaccine designed by IrsiCaixa, in collaboration with the Germans Trias i Pujol University Hospital (HUGTiP) and within the framework of the HIVACAT program, is showing encouraging results on its path towards a potential cure.
Read more
Rutipaste: First GLP-compliant multicentre study led by IGTP researchers at CMCiB
The first multicentric GLP study led by a research team from the Germans Trias i Pujol Research Institute (IGTP) has been conducted at CMCiB facilities to validate the efficacy and safety of Rutipaste, an innovative product designed to treat anastomotic leakage (AL) following colorectal surgery.
Read more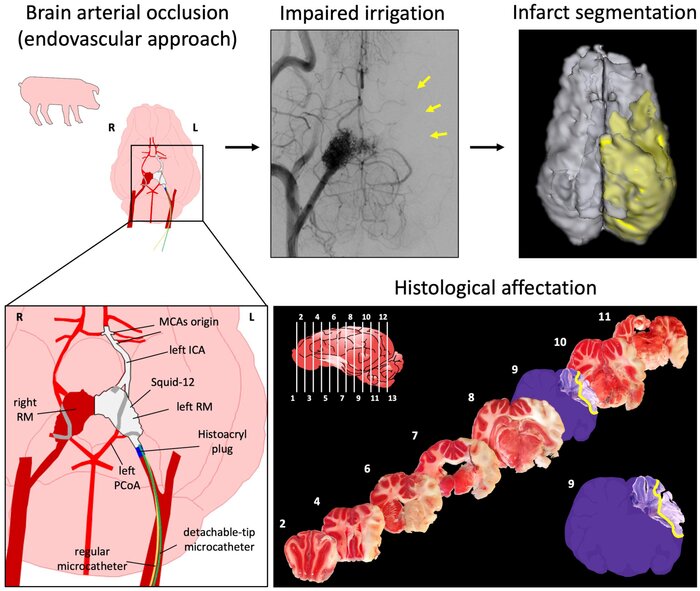
Establishment of a new reproducible and minimally invasive stroke model through an endovascular approach
Researchers of the Cellular and Molecular Neurobiology (CMN) Research Group at the Germans Trias i Pujol Institute (IGTP) have developed and established a novel, reproducible and minimally invasive stroke model in pigs through an endovascular approach.
Read more
NIMBLE Diagnostics: Groundbreaking Microwave-based Medical Device for non-invasive stent monitoring
NIMBLE Diagnostics is pioneering a groundbreaking microwave-based medical device for the non-invasive monitoring of patients with implanted stents.
Read more